LECTURES 15, 16, 17
Heat exchangers
Heat exchangers are devices where two moving fluid streams exchange heat without mixing.
Heat is transferred from the
hot fluid to the cold one.
Under steady operation, the mass
flow rate of each fluid stream flowing through a heat exchanger remains
constant.
w = 0, Dke = 0, Dpe = 0
Heat exchangers are intended
for heat transfer between two fluids within the device.
Usually, the entire heat
exchanger is selected as the control volume and Q becomes zero.
Problem:
Refrigerant-134a is to be cooled by water in a condenser. The refrigerant
enters the condenser with a mass flow rate of 6 kg per min at 1 Mpa and 70oC
and leaves at 35oC. The cooling water enters at 300 kPa and 15oC
and leaves at 25oC. Neglecting any pressure drops, determine (a) the
mass flow rate of the cooling water required and (b) heat transfer rate from
the refrigerant to water.
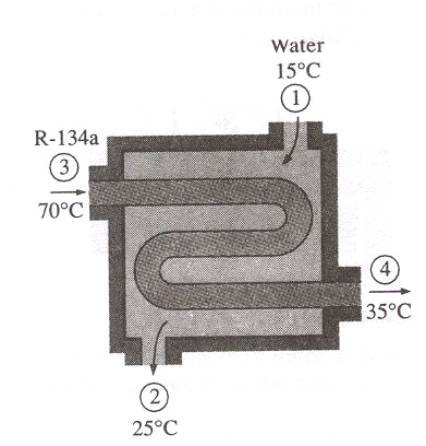
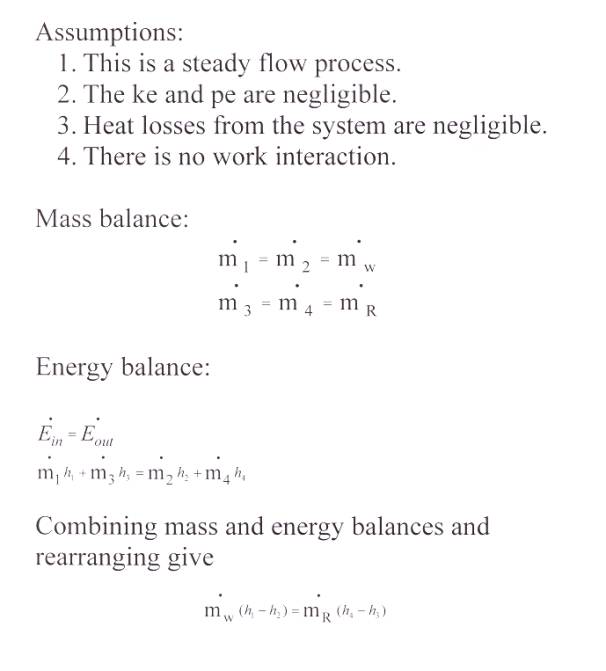
Solution:
Take the entire heat exchanger
as the control volume.
h1 = hf@15C
= 62.99 kJ/kg
h2 = hf@25C
= 104.89 kJ/kg
The refrigerant enters the
condenser as a superheated vapor and leaves as a compressed liquid at 35oC.
P3 = 1MPa, T3
= 70oC, h3 = 302.34 kJ/kg.
P4 = 1MPa, 35oC, h4 = hf@35C = 98.78
kJ/kg
Substituting,
mw (62.99
104.89) = 6(-302.24)
mw = 29.15
kg/min
(b) Heat transfer
from the refrigerant to the water:
Choosing volume occupied by
the water as control volume,
Qw,in + mw
h1 = mw h2
Qw,in = mw(h2
h1) = 29.15 (104.89 62.99)
= 1221 kJ/min
Mixture chambers
Mixing of two streams of fluids is common in engineering applications.
The conservation of mass for
a mixing chamber requires inflow rate to be equal to outflow rate.
w = 0, q = 0, ke = 0, pe = 0
The conservation of energy equation is similar to conservation of mass equation, i.e., energy influx in the control volume is equal to energy outflux from the control volume.
Problem: A small plant
has a boiler which produces superheated steam at 3 MPa and 300oC. It
is necessary for a particular process to have saturated steam available at 2
MPa. It is possible to desuperheat the superheated steam by spraying cold water
on to it. Suppose the superheated steam enters such a desuperheater at the rate
of 1 kg/sec, determine the rate at which spray water at 30oC and 3
MPa is to be added in the desuperheater.
![]()
2 Water, 30oC
![]()
![]()
 1 3
Sat.
Steam 2MPa Sup. Steam
1 3
Sat.
Steam 2MPa Sup. Steam
3MPa, 300oC
Mass balance: ![]()
Energy balance: ![]()
h1 = 2995.2
kJ/kg, h2 = 125.6 kJ/kg
h3 = 2797.3
kJ/kg, m1 = 1 kg/s
m3 = 1+m2
2995.2 + m2
(125.6) = (1+m2)2797.3
m2 = 0.0741 kg/s
Throttle
Throttle is any kind of flow restricting device that causes a significant pressure drop in the fluid.
The pressure drop does not
involve any work.
A large drop in temperature
often accompanies the pressure drop in the fluid.
Throttling devices are commonly
used in refrigeration and air-conditioning applications.
q = 0, w = 0, Dke = 0, Dpe = 0
Hence across a steady flow
throttling device,
h2 = h1
Internal energy + flow energy = constant
Joule-Thomson Coefficient
The temperature behaviour of a fluid during throttling process is described by Joule-Thomson coefficient.
m = (ΆT/ΆP)h
Joule-Thomson coefficient is a measure of the change in temperature with pressure during a constant enthalpy process.
mJT <0,temperature increase as the pressure drops
=0, temperature remains constant
>0, temperature decreases


Example:
Steam at 800 kPa, 300oC is throttled to 200 kPa. Changes in ke are negligible for this process. Determine the final temperature of the steam, and the average Joule-Thomson coefficient.
Control volume: Throttle valve
hi = he
Since he = hi = 3056.5 kJ/kg, and Pe = 200kPa,
These two properties determine the final state.
From superheat table for steam, Te = 292.4oC
mJT (ave) = (DT/DP)h = -7.6 / -600
= 0.0127 K/kPa
Throttling calorimeter
When wet steam is throttled to a low pressure (usually atmospheric), steam becomes superheated.
The temperature of the superheated
steam is measured.
Knowing the pressure and
temperature of the steam in the calorimeter, its enthalpy can be determined.
he= hi
= X hg + (1-X)hf
Knowing the value of hg and hf at inlet pressure,
X can be calculated from the above relation.

Example:
A throttling calorimeter is
used to measure the quality of wet steam in a pipe carrying steam at 2 MPa. The
pressure and temperature in the calorimeter are measured as 0.05 MPa and 150oC.
Determine the quality of steam in the mains.
Throttling process is an
isenthalpic process. Therefore,
hi = he
= 2780.5 kJ/kg
2780.5 = hf + X hfg
= 908.4 + X (1888.9)
or, X = 0.9911
Uniform flow processes
Some unsteady flow processes
can be reasonably represented by simplified model, the uniform flow
processes.
1. At any instant
during the process, the state of the control volume is uniform (it is the same
throughout). The state of the control volume may change with time but it does
so uniformly.
2. The fluid
properties may differ from one inlet or exit to another, but the fluid flow at
an inlet or exit is uniform and steady.
Charging of a cylinder
(mf mo)
hi = mf uf mo uo
Further, if the cylinder is
initially evacuated, the equation reduces to
hi = uf
Discharge of a cylinder
(mo mf)
(h + V2/2) = Q + mo uo mf uf
Example:
A rigid insulated tank that is initially evacuated is connected through a valve to a supply line that carries steam at 1MPa and 300oC. Now that valve is opened and the steam allowed to flow slowly into the tank until the pressure reaches 1MPa, at which point the valve is closed. Determine the final temperature of the steam in the tank.

Solution:
We take the tank as the system. This is a control volume since mass crosses the system boundaries during the process. This is an unsteady flow process since changes occur within the control volume. For an evacuated tank, mo = 0 and mo uo = 0. There is only one inlet and no exit.
Assumptions:
1. Uniform flow process. The properties of steam entering the control volume remain constant during the entire process.
2. The ke and pe terms are zero for the tank since it is stationary.
3. The ke and pe of the stream are negligible.
4. w = 0, q = 0
Mass balance:
mf mo = Dmsystem
mo = 0
Energy balance:
Ein
Eout = DEsystem
moho
= m2u2 (since W = Q = 0, ke = pe = 0 and
mo = 0)
Combining the
mass and energy balances,
uf = hi
At Pi = 1MPa and Ti = 300oC, hi
= 3051.2 kJ/kg
Which is equal to uf.
Using steam table, at Pf = 1MPa and uf = 3051.2
kJ/kg, Tf = 456.2oC.
Alternative solution:
Consider the region within the tank and the mass that is destined to enter the tank as a control mass system as shown in the figure. Since no mass crosses the boundaries, viewing this a control mass system is appropriate.
During the process, the steam upstream (the
imaginary piston) will push
the enclosed steam
in the supply
line into the tank at a constant
pressure of 1MPa.
Then the work done on the
system during
this process is
W = -Pi (Vf Vi)
= -Pi (Vtank (Vtank+Vi))
= PiVi
where, Vi is the volume
occupied by steam before it enters the tank and Pi is the pressure at
the moving boundary (the imaginary piston face)
The energy balance for the control mass system gives
mi Pi vi = mf uf mi ui
uf = ui +Pi vi
= hi
Example:
A pressure cooker has a
volume of 6 litres and an operating pressure of 75 kPa gage. Initially, it
contains 1 kg of water. Heat is supplied to the cooker at a rate of 500 W for
30 minutes after the operating pressure is reached. Assuming an atmospheric
pressure of 100 kPa, determine,
(a) the temperature
at which cooking takes place
(b) the water left in
the pressure cooker at the end of the process.
Solution:
Take the pressure cooker as
the system. This is a control volume system since mass crosses the system
boundaries during the process. This is an unsteady process since changes occur
within the control volume.
Assumptions:
1. Uniform flow
process.
2. ke and pe are
negligible for the escaping steam.
3. Within the
pressure cooker, Dke and Dpe are zero. Therefore, DEsystem
= DUsystem
4. The pressure (and
thus the temperature) in the cooker remains constant.
5. Steam leaves as a
saturated vapor at the cooker pressure.
6. There is no work
involved.
7. Heat is
transferred to the cooker at constant rate.
(a) The absolute pressure
within the cooker is Pabs =
Pgage + Patm = 75 kPa + 100kPa = 175kPa
T = Tsat@175 kPa = 116.06oC
(b)
Mass balance:
me = (mf
mo)cv
Energy balance:
Ein Eout
= DEsystem
Qin-mehe
= (mfuf mouo)cv
Combining mass and energy
balance,
Qin =
(mf mo)he + (mfuf mouo)cv
The amount of heat transferred during this process is
Qin = (0.5)(30)(60)
= 900 kJ
he = he@175kPa
= 2700.6 kJ/kg
The initial internal energy
is found after the quality is determined:
vo = V/mo
= 0.006/1 = 0.006 m3/kg
xo = (vo-vf)/vfg
= (0.006 0.001)/(1.004 0.001)
= 0.005
thus,
uo = uf + xoufg = 486.8 + (0.005)(2038.1)
= 497.0 kJ
The mass of the system at
the final state is
mf = V/vf
substituting this in the
energy equation,
Qin = (mo
V/vf)he + {(V/vf)uf mo uo}
There are two unknown in
this equation. Assuming saturation conditions exists in the cooker,
vfinal = vf
+ xf vfg = 0.001 + xf (1.004 - 0.001)
ufinal = uf
+ xfufg = 486.8 +xf(2038.1)
Substituting the above in
the energy equation, xf becomes the only unknown.
xf = 0.009
Thus,
vfinal = 0.001 +
(0.009)(1.004 - 0.0001)
= 0.010 m3/kg
mfinal = V/vfinal
= 0.006 / 0.01 = 0.6 kg
Therefore, after 30 min, 0.6 kg of water is remaining in the pressure cooker.